|
Releasing energy stored within molecules can have powerful effects: steam bursts from the hard popcorn kernel creating a tasty snack; deploying an airbag can save your life in a collision; and explosives can shift thousands of tonnes of material in an instant.
|  |
|
1,2,3-Triazoles are cyclic molecules containing five atoms, of which three are adjacent nitrogen atoms. In general, triazoles are stable and successfully used as a way of efficiently joining two molecular fragments together. However, in 1-sulfonyl triazoles: 1-STs, we use a sulfonyl group to carefully tune the reactivity profile. We are developing new reactions so that a tiny amount of catalyst can promote controlled loss of two of the nitrogen atoms from the 1-ST as nitrogen gas and capture the associated energy directing it towards formation of complex products with exquisite control and efficiency. | 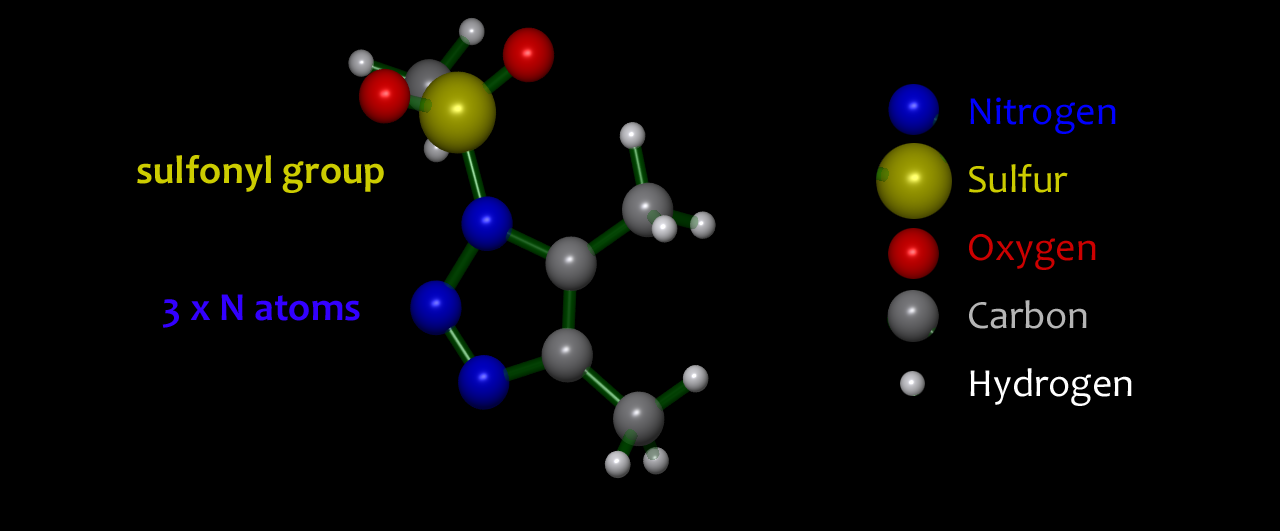 1-sulfonyl-1,2,3-triazoles: 1-STs |
|
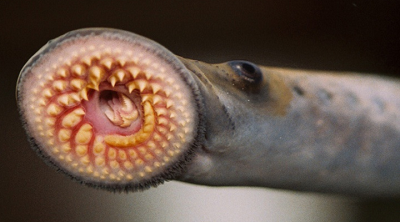
We have applied this strategy to design new and efficient synthesis of tetrahydrofurans: important molecules found at the heart of many bioactive compounds [see publication 12, publication 13]. We then applied this research to complete the first ever synthesis of petromyroxol, a natural molecule produced by the sea-lamprey [see publication 15]. This molecule could play an important part in the way that the sea lamprey finds its way back from open water to rivers to breed and we produced a significant amount of petromyroxol which unlocks the possibility for further study of this fascinating creature and its marine biology. Prof. Weiming Li who is a world-expert in the sea lamprey has more information on his group's website [Li Research Group, Michegan State University]. |  compounds made from 1-STs using this novel strategy |
|  |
| Sea Lamprey | petromyroxol |
|
We are continuing to extend this strategy to create bioactive compounds, novel powerful catalysts and next-generation organic materials. | |